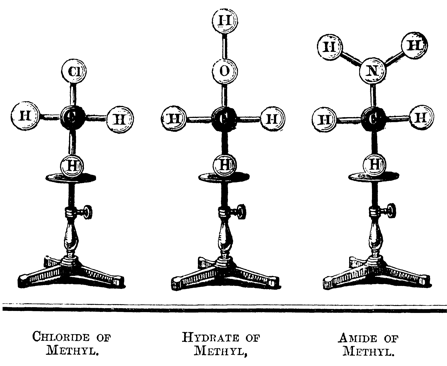
 Figure 52: "Paper tools" and 3D-models in chemistry in the 19th century
Figure 52: "Paper tools" and 3D-models in chemistry in the 19th century
For illustrations see:
„History of the molecule“ in Wikipedia
content
„Paper tools“ as productive tools
Three-dimensional models for communicating and teaching
„Paper tools“ as productive tools
There were several attempts to find a notation for atoms and their bonds since René Descartes’ Hook-and-eye-model (1625). New ideas have been brought in by William Higgins (1789) and John Dalton (1808). In 1833 Marc Antoine Auguste Gaudin introduced „volume diagrams“ of molecules.
How the chemists Justus Liebig and Jean Dumas constructed „models“ - i. e. notations of structures on paper - in the years 1832-40 is described by Ursula Klein (1999, 153-164). Later (2003, 2-3; similar 118) she analyzes more elaborately the use of sign systems and their manipulations on paper in the laboratory sciences of chemists in the early 19th century.
Ursula Klein found out
„that chemists began applying chemical formulas not
primarily to represent and to illustrate preexisting knowledge, but rather as
productive tools on paper or ‚paper tools’ for creating order in the jungle
of organic chemistry. Berzelian chemical formulas ... were tools for
experimentally investigating organic chemical reactions and for constructing
models of reactions and of the invisible constitution of organic substances ...
The manipulation of formulas on paper and the visual display of possible
recombinations of signs had the suggestive power of introducing new
significances, which chemists attempted to match up with experimental traces.
In doing so, they tacitly modified the existing intellectual framework and
introduced new concepts and research objects that opened up new avenues of
research, such as the experimental practice of synthesizing archipelagos of
organic derivates by means of ‚substitutions’“ (2003, 2-3).
In his Heidelberg lectures of 1857/58 the German chemist August von Kekulé introduced his „bread rolls“ or „sausage formulae“ (Christoph Meinel, 2004, 258). A year later the Scotsman Archibald Couper proposed the graphic visualization by the structural formula with valence lines.
Much more sophisticated drawings of molecules were published by the Viennese teacher Joseph Loschmidt in 1861. In the same year in Edinburgh a medical graduate, Alexander Crum Brown, suggested drawing circles around the atomic letters or labels. The „graphic formulae“ proposed by Edward Frankland and Frank Baldwin Duppa in January 1867 resemble the ones used today.
Three-dimensional models for communicating and teaching
According to the historian of chemistry Christoph Meinel already the English teacher John Dalton around 1810 made atomic models using wooden balls of various sizes (2004, 270, footnote 1; see also 256, 242).
Nearly half a century later the German chemist August Kekulé constructed and used 3D-models of molecules in 1857 in Heidelberg (Christoph Meinel, 2004, 259, see also 258), later in Ghent and since 1867 in Bonn - "because of an irresistible need of visualization" (Leopold Horner, 1965, 240). In his „Lehrbuch der Organischen Chemie“ (vol. 1, 1861, 157f) he writes:
„Es ist an sich einleuchtend, dass man die Stellung der Atome im Raum, selbst wenn man sie erforscht hätte, nicht auf der Ebene des Papiers durch nebeneinandergesetzte Buchstaben darstellen kann; dass man vielmehr dazu mindestens einer perspectivischen Zeichnung oder eines Modelles bedarf. Dass man aber durch das Studium der Metamorphosen die Lagerung der Atome in der bestehenden Verbindung nicht ermitteln kann, ist ebenfalls klar …“
To clarify the real occurrences in respect to “Kekulé’s dreams” - resp. the discovery of the benzene ring - John H. Wotiz of Southern Illinois University made an exhaustive study of the documents and lore the scientist left to his heirs. In April 1990 Wotiz organized a symposium of chemists and scholars to try to sort fact from fiction in assessing Kekulé's career (John H. Wotiz, 1993).
In the meantime also the German chemist August Wilhelm von Hofmann - since 1845 teaching for 20 years in London, then moving to Berlin - started constructing two different kinds of visualizations:
· „type-moulds“ (1862), wire frames into which little boxes could be put, and little tin boxes as "a simple mechanical contrivance" to represent volumes and demonstrate types and reactions (Christoph Meinel, 2004, 245; with an illustration Christoph Meinel, 2008, 223)
· „glyptic formulae“ (1865) from balls and wires. „To exhibit the ‚combining powers’, i. e. the valencies, metallic tubes and pins were screwed into the balls ‚to join the balls and to rear in this manner a kind of mechanical structures in imitation of the atomic edifices to be illustrated’“ (Christoph Meinel, 2004, 250; illustration 251).
August Wilhelm Hofmann: On the combining power of atoms. Proceedings of the Royal Institution of Great Britain 4, 1865, 401-430, the 3D- models are called „glyptic formulae“ 426; reprinted in Chemical News 12, 1865, 166-190.
Christoph Meinel (2004, 243, 265, 270) denies that the molecular models of that time were illustrating theoretical concepts. He argues that a new kind of modelling was invented
„not primarily to express chemical theory,
but rather as a new way of communicating a variety of messages. By
manipulating tin boxes or tinkering with little spheres and toothpicks,
chemists not only visualized their abstract theoretical notations but also impressively
testified to the claim that they would build a new world out of new material“
(2004, 243).
“The chemists prepared 3-D models for teaching purposes, and in using them they
learned to link the mind’s eye with theoretical notions, with the manipulating
hand, and with laboratory practice“ (2004, 265).
Meinel states that these early molecular models were looked upon with suspicion by many a scientist and therefore were used mostly for lecture illustration. They „appeared in the context of teaching, not research“ (2004, 256).
Only when Kekulé had improved a „brass bar“-model by James Dewar (Christoph Meinel, 2004, 248-249) in 1867 his molecular models „turned into research tools that could be used to interpret and guide chemical reactions“.
“Once chemists had become used to this form of representation and learned to read spatial meaning, the new model could be applied to understanding unknown mechanisms and predicting possible reactions by giving them visual plausibility” (Christoph Meinel, 2004, 262; illustration 263).
The final consequences of the fourfould valency of the carbon atom were drawn 1874 by Jacobus Hendricus van't Hoff and Joseph Achille Le Bel. As in physics these attempts were laughed at as „aberrations“ or „fanciful plays“. But this stereochemistry stood the test and in 1885 resp. 1890 Adolf von Baeyer and Hermann Sachse were able to gain new insight by means of tetrahedral constructed wire models resp. folded pieces of paper and verify with experiments.
In 1934 Herbert Arthur Stuart introduced the calotte model (or: space-filling model - see: Leopold Horner, 1965, 242).
On the productive function of three-dimensional molecular models as representational tools, see Erich Francoeur (1997, 7-40).
Bibliography
model: special topics - Anschaulichkeit in der Chemie
 Dr. phil. Roland Müller, Switzerland / Copyright © by Mueller Science 2001-2016 / All rights reserved
Dr. phil. Roland Müller, Switzerland / Copyright © by Mueller Science 2001-2016 / All rights reserved
Webmaster by best4web.ch